Animal Testing: What Was It Ever Good For?
Researchers develop alternatives to animal-testing models, but old-guard institutions hang on to inefficient traditions.
|
Listen To This Story
|
Legions of animal rights activists and scientists are making strides in their battles against animal testing in biomedical research with the help of cutting edge technology — even as the transition to animal-free research is challenged by established institutions and Washington, DC lobbyists.
“There’s a bias toward using animals in labs that’s been hardwired into scientific training,” said Ryan Merkely, Director of Research Advocacy at Physicians Committee for Responsible Medicine. PCRM focuses on education and advocacy toward new research methods that they say are more applicable to humans than animal research. “We encourage researchers to question whether it’s justifiable to conduct animal experiments when the practice has so often failed to improve human health.”
PCRM community member Melanie Ort is a biomedical scientist who researches human bone marrow regeneration. A lot of her work requires modeling, but she won’t use animals for her research. Ort argues that experimentation on animals is cruel, wasteful, and wildly expensive: It’s estimated that more than $28 billion of taxpayer-funded animal research performed on mice, birds, dogs, cats, and monkeys in the US every year is wasted because results are often not replicable in people during clinical trials.
“As a result of almost a hundred years of animal testing, we know every biomarker in mice, yet we haven’t found most of the equivalents in humans,” said Ort from her office in Berlin, Germany. She added that animal studies are not only frequently inapplicable to humans, they can also lead to dangerous and unhealthy public misinformation.
Ort points to the collection of countless news stories tracked by the X account “Just Says in Mice” which mocks news-media hype around the medical research findings in animals that usually don’t translate to people. One such story, in The Independent, featured a mouse study that found a high intake of Pepsi or Coca-Cola could increase testicle growth and testosterone. “If we were all mice,” as a Harvard Medical School post puts it, “Alzheimer’s disease, cancer, diabetes, and most inherited disorders would be a thing of the past.” Or, if the mice model was predictive for humans, men could also increase their testosterone with sugary soda, but a study in human males found the opposite effect on men: sugar-sweetened beverages clearly correlated with lowered testosterone.
Ort, a bioethics advocate who’s been against animal testing from the start of her career 13 years ago, says that it can be difficult to make progress in research without animal testing. As a young visiting researcher at a top-tier university in the US a decade ago, her refusal to use animals for tests and organ harvesting made her work difficult. (Ort asked that we not name the schools where she’s performed research because she doesn’t want to “make enemies with people in the field” and is weary of animal-testing lobbying groups that can be intimidating.)

“They told me that ‘This is the gold standard, you need to get over killing mice or you’re out,’” she said. “The more established researchers don’t want to leave a winning team, because if you’ve been publishing animal research findings for the past 40 years in Nature, you’re not going to want to switch and negate all of the work that you did in your life.”
But Ort instead went on to develop award-winning alternatives to animal testing, including 2D and 3D methods for modeling musculoskeletal regeneration in humans. She’s now teaching non-animal methods to other PhD students. Last October, Ort and her peers published a guide “to be used by authors who use non-animal methods to avoid and respond to animal methods bias from manuscript reviewers.”
Meanwhile, at the Wyss Institute for Biologically Inspired Engineering at Harvard University, founding director Donald Ingber, M.D., Ph.D., and his peers have pioneered and commercialized one of the leading animal-testing alternatives through a company called Emulate. Emulate developed Organs-on-Chips: silicone rubber chips smaller than a domino, lined with living human cells that emulate human organs. Ingber says Organs-on-Chips have helped researchers understand the body’s responses to myriad drugs.
By eliminating the need for animal testing, Ingber says that Emulate’s technology is enabling research that’s more relevant to humans, which can transform how drugs are developed. Right now, the pharmaceutical industry is estimated to spend anywhere from $314 million to $2.8 billion in order to bring a drug to market, a significant portion of which is spent on animal experiments, known as preclinical trials. One report published in the Journal of Health Economics found that this animal-based preclinical research-and-development totals $474 million for each new drug, or approximately 31 percent of a company’s total expenses on drug development. In the pharmaceutical industry, animal trials cost anywhere from 1.5 to 30 times more than non-animal, in-vitro research.
“The power of chips is not necessarily that they reduce the preclinical trial time versus a particular animal study, but rather that they will produce much more human relevant predictive information,” Ingber wrote in an email to WhoWhatWhy. Ingber says a major advantage of these chips is the ability to personalize them for individuals or groups, “female versus male, old versus young, patients with particular disease processes and symptoms, etc. All of this will increase the likelihood of success in the clinic, which will be good for patients and reduce costs dramatically, plus shorten time because of reduced failure rate.”
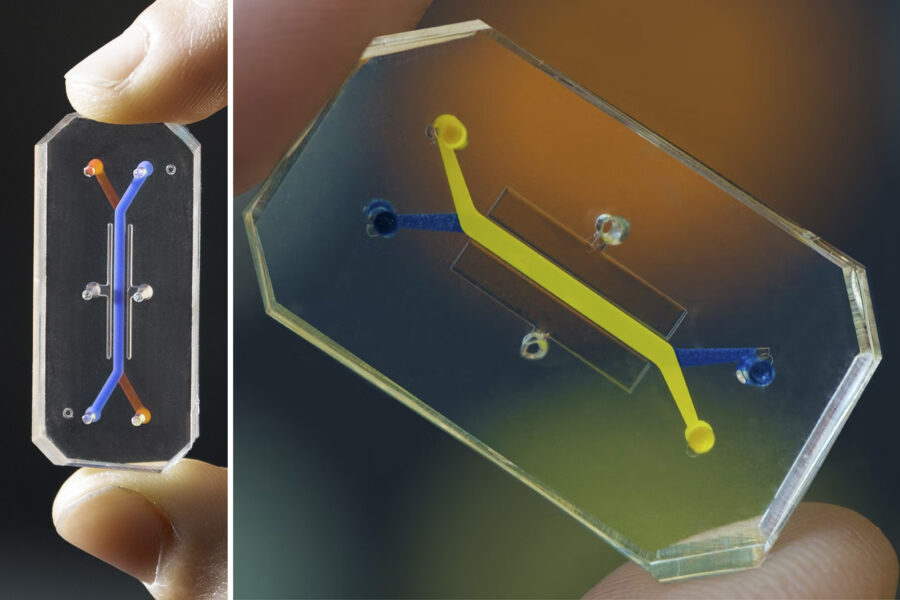
Ingber’s team has published multiple Organs-on-Chips studies touting the success of the technology, including development of an airway-on-a-chip that mimics living lung tissues and can help screen drugs against coronavirus, and a liver chip that peer-reviewed research confirmed can assess drug toxicities more quickly and safely than animal studies on the organ — all without the need for traditional animal research.
Last November, Moderna presented a webinar in which the pharmaceutical company detailed how it used Emulate’s liver chips (instead of monkeys) to test for potential toxicities related to the components of RNA therapeutic vaccine delivery. Ingber shared Moderna’s findings related to the company’s considerable cost and time savings with WhoWhatWhy: Using Emulate’s chips, the testing will cost $325,000 and take four to five months, as opposed to a standard nonprimate testing method, which would cost $5.25 million and require 60 months.
Yet, while some of his work has been celebrated by organizations like Moderna and the NIH, Ingber must take on the role of both researcher and advocate in order to make progress in the field. In a paper published in 2020 by Ingber in Advanced Science, he addressed publishing reviewers’ biases for animal studies, suggesting that they should give more consideration to human organ-on-a-chip studies and citing the myriad animal-testing shortfalls, including ethical concerns around using animal models. He concludes “that results from preclinical animal models frequently fail to predict drug responses in humans.”
It’s not that researchers who advocate for animal testing models are primitive, naive or sadistic; they’re just following the inertia in the system they’ve come to know, while gingerly testing the potential limitations of organs-on-chips, and staying within the bounds of the Food, Drug, and Cosmetic Act. Created in 1938 and only recently replaced by new legislation, the act required all new drugs and medical devices to prove their safety in a course of animal testing before they could move onto human clinical trials.
Opponents to animal testing have plenty of data to support their objections. Aspirin, a wonder drug for people, is toxic for animals. Meanwhile, over 90 percent of drugs and vaccines that work in animals — including HIV vaccines that were found to be effective in chimpanzees — do not work for humans.
With all this data, why is animal testing still so popular? Animal welfare researcher Eric Kleiman suggests the answer can be found by following the money.
Policy lobby groups like the National Association for Biomedical Research (NABR) and National Academies of Sciences, Engineering, and Medicine have been sounding alarms about the post-Covid shortage of long-tailed macaque monkeys available for research experiments. Kleiman points out that NABR’s board members include some big players in animal research. One is John Sagartz, who serves as the chief strategy officer for a corporation that owns Envigo, a controversial beagle production facility where hundreds of puppies and dogs bred for medical experiments were found dead, dehydrated and mistreated before the facility was shut down by the US Department of Justice in 2022. Another is Laurie Brignolo, executive director of the animal care program at U.C. Davis, which made headlines in 2023 after Elon Musk’s brain chip company Neuralink performed a number of deadly experiments on monkeys at the university, that led to a USDA probe on the company’s violations of the Animal Welfare Act.
“These for-profits, like Charles River Labs, Inotiv, and Labcorp, aren’t going to meekly go away if they can make significant revenue using live animals in experimentation,” said Kleiman. “You have to address the financial incentives if non-animal alternatives are going to succeed in this profit driven system.”
Despite the Biden Administration’s FDA Modernization Act 2.0, which removed the animal trial mandate from drug tests in 2022, industry players continue to advocate in favor of continuing experiments on mammals, particularly nonhuman primates and monkeys.
This February, NABR issued almost a dozen press releases in multiple languages to promote its petition against the International Union for Conservation of Nature, which designated long-tailed macaques as “endangered” and “vulnerable” after poachers in Southeast Asia decimated populations across the region in recent years.
While monkeys are in short supply, researchers continue to use more than 100 million animals in experiments every year in the US, with similarly poor outcomes, according to the PCRM.
Last November, PCRM authored a letter urging the National Institutes of Health’s new director, Monica Bertagnolli, to stop funding unnecessary animal studies that cost taxpayers nearly $50 billion each year. The letter argues that the NIH’s current “gold standard” of animal experiments “contributes to failures and wasteful spending in the drug development pipeline and puts clinical trial participants at risk by failing to capture unsafe or ineffective products.” Signed by 246 scientists, physicians, ethicists, and advocates, the letter urges Bertagnolli to redirect research funding to new technologies, like organ chips, that can “reliably mimic human biology and clinical responses” more quickly, cheaply, without using animals.
“In the majority of these studies, there’s no justification for using these animals,” said the PCRM’s Ryan Merkley. “Most of them will be used and killed without any benefit to humankind at the end of it.”





